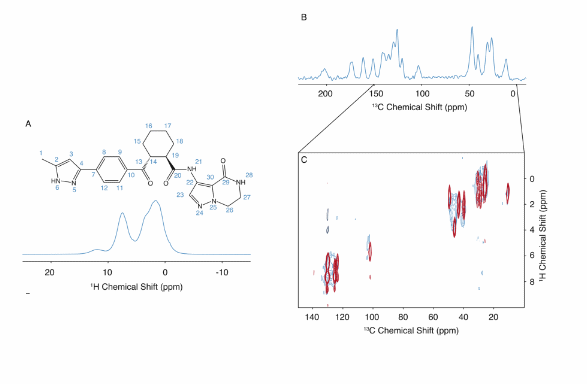
Challenge: The atomic-level structure of amorphous drug forms is often difficult to determine, yet understanding these structures is critical for optimizing drug stability and performance. For the drug atuliflapon, a potent FLAP (5-lipoxygenase activating protein) inhibitor, the lack of crystallinity complicates the use of traditional structural analysis techniques, making it challenging to identify the interactions that stabilize its amorphous form.
NMR Solution: Using chemical-shift-driven NMR crystallography, academic researchers in collaboration with AstraZeneca were able to map the complete atomic-level structure of amorphous atuliflapon. This advanced NMR approach identified specific hydrogen-bonding motifs and the preferred conformations of key molecular components, such as the cyclohexane and pyrazole rings. These conformations and interactions play a critical role in stabilizing the drug’s amorphous form, including hydrogen bonds with water molecules and optimized intermolecular interactions.
Impact: This study demonstrates how NMR crystallography provides essential insights into the stabilization mechanisms of amorphous drug forms, which are vital for improving drug formulations. The ability to uncover detailed conformational preferences and intermolecular interactions at the atomic level opens new possibilities for the rational design of more stable and effective pharmaceutical compounds. For industries developing amorphous drug formulations, this approach is a game-changer, offering a deeper understanding of drug stability that could enhance both efficacy and shelf-life.
Key Points for industry:
- Stabilizing Amorphous Drugs: Atomic-level insight help in understanding how hydrogen bonding and molecular conformations stabilize amorphous drug forms.
- NMR Crystallography: This method offers precise structural information, even for non-crystalline substances, helping to optimize drug formulation.
- Improved Drug Performance: The findings enable better stability and longer shelf-life for amorphous drugs, ensuring enhanced pharmaceutical efficacy.
Reference: Holmes, J. B.; Torodii, D.; Balodis, M.; Cordova, M.; Hofstetter, A.; Paruzzo, F.; Lill, S. O. N.; Eriksson, E.; Berruyer, P.; Almeida, B. S. de; Quayle, M.; Norberg, S.; Ankarberg, A. S.; Schantz, S.; Emsley, L. Atomic-Level Structure of the Amorphous Drug Atuliflapon via NMR Crystallography. Faraday Discuss. 2024.
https://doi.org/10.1039/D4FD00078A